In the past, most chemists had difficulty separating elements which were similar to each other. Elements with similar properties couldn’t be separated until the tests in the 18th century. Early chemists believed that metals grew in the planet, in the same way that plants grow. In addition, unattractive metals were thought to be immature or young metals, such as lead, and more attractive metals were thought to be partially grown, such as tin.
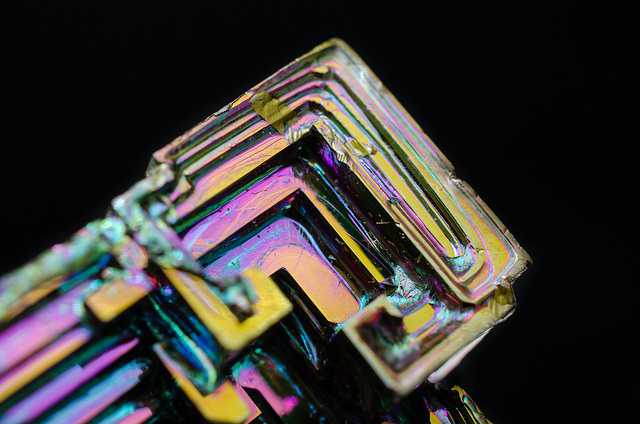
Gold and silver were the most mature metals. For this reason, the identification was very difficult. Bismuth was not an exception, since this element was also confused with other elements. According to old manuscripts, bismuth was often confused with antimony, tin, lead, or even silver. It is hard to say who exactly discovered bismuth, just like with antimony and arsenic. It is thought that the name bismuth was taken from two German words, weisse masse, which means white mass.
These words describe how this element appears in nature. Before bismuth came into common use, the name was shortened to wismuth, and then to bisemutum. This element has a number of uses, and in this article we will mention all the important uses of bismuth.
Uses
Bismuth is used in many different applications. However, pure metallic bismuth has just a few uses. The majority is consumed in bismuth alloys, and in chemicals and pharmaceuticals. The remainder is used in catalysts, paints, ceramics, and a variety of minor applications. There are many reasons why alloys of bismuth are useful: When they are solidify (freeze), bismuth and many of its alloys expand slightly. This allows the bismuth to form a perfectly sharp replica of the mold by filling all corners of a mold. This is a valued property when used in plumbing or soldering.
A lot of bismuth alloys have a low melting point, often even below the temperature of boiling water. Therefore, a bismuth-alloy casting can be covered with certain materials, such as plastic, to form an intricate machine part. Furthermore, the bismuth-alloy core is then removed easily by melting it in hot water and pouring it out. The low-melting bismuth alloys use is common in sprinkler systems in buildings. The sprinkler becomes unplugged and water sprays the fire when the alloy melts in fire-heated air.
Each year in the United States, this application accounts for over one-third of the bismuth use.Bismuth metal is relatively non-toxic and inert, and has replaced toxic lead in a number of applications, such as metal alloys, birdshot, plumbing, soldering, bullets, and other applications. Most bismuth alloys are relatively malleable and soft. This means that bismuth can be hammered into thin sheets and then alloyed with iron to create malleable irons.
In addition, bismuth compounds are used in stomach-upset medicines, cosmetics, treatment of stomach ulcers, and soothing creams. There are many applications in which industry uses bismuth. It is a catalyst in the production of acrylic fibers. Since bismuth in non-toxic, it replaces lead in some ceramic glazes and paints.